Objectives
In RNA-Seq, the abundance level of a gene is measured by the number of reads that map to that gene. Once the reads have been mapped to our reference, we must now count the number of reads that map to RNA units of interest to obtain gene/exon/transcript counts. Here, we shall look at different methods for doing this.
Count reads mapping to genes
Get set up
cds cd my_rnaseq_course cd day_3_partA/gene_counting_exercise
Bedtools
Bedtools is a great utility for working with sequence features and mapped reads in BAM, BED, VCF, and GFF formats.
We are going to use it to count the number of reads that map to each gene in the genome. Load the module and check out the help for bedtools and the multicov specific command that we are going to use:
module load biocontainers module load bedtools type bedtools
The bedtools multicov command takes a feature file (GFF) and counts how many reads are in certain regions from many input files. By default, it counts how many reads overlap the feature on either strand, but it can be made specific with the -s option.
Note: Remember that the chromosome names in your gff/gtf file should match the way the chromosomes are named in the reference fasta file used in the mapping step. For example, if reference file used for mapping contains chr1, chrX etc, the GFF/GTF file must also call the chromosomes as chr1, chrX and so on.
Let's double check this using grep.
grep '^>' ../reference/genome.fa
cut -f 1 ../reference/genes.gtf |sort|uniq -c
Let's fix the one chromosome that is differently named in the gtf file- mitochondrion.
sed 's/^dmel_mitochondrion_genome/M/' ../reference/genes.gtf > ../reference/genes.formatted.gtf cut -f 1 ../reference/genes.formatted.gtf |sort|uniq -c
In order to use the bedtools command on our data, do the following:
Warning: To submit to queue
nano commands.bedtools
#enter below commands into the file
singularity exec ${BIOCONTAINER_DIR}/biocontainers/bedtools/bedtools-2.27.1--1.simg bedtools multicov -bams hisat_results/C1_R1.sorted.bam hisat_results/C1_R2.sorted.bam hisat_results/C1_R3.sorted.bam hisat_results/C2_R1.sorted.bam hisat_results/C2_R2.sorted.bam hisat_results/C2_R3.sorted.bam -bed ../reference/genes.formatted.gtf > gene_counts.gff
#use ctrl+x to quit out of nano
launcher_creator.py -j commands.bedtools -n multicov -q normal -t 02:00:00 -a UT-2015-05-18 -l bedtools_launcher.slurm -m "module load biocontainers; module load bedtools" sbatch bedtools_launcher.slurm
HTseq
HTseq is another tool to count reads. bedtools has many many useful functions, and counting reads is just one of them. In contrast, HTseq is a specialized utility for counting reads. HTseq is very slow and you need to run multiple command lines in order to do the same job as what bedtools multicov did. However, if you are looking for more fine grained control over how to count genes, especially when a read overlaps more than one gene/feature, htseq-count would be an option.
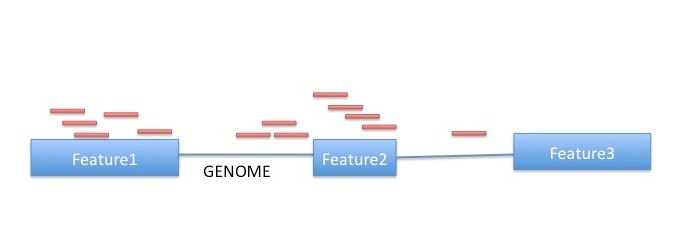
htseq-count has three modes for handling overlaps:
- mode union. This mode is recommended for most use cases.
- mode intersection-strict.
- mode intersection-nonempty.
Image from:http://www-huber.embl.de/users/anders/HTSeq/
Htseq is a module on stampede2, but not part of biocontainers. Module spider htseq shows us the other modules (intel/17.0.4) we need in order to load htseq.
module spider htseq module load intel/17.0.4 module load htseq htseq-count -h
IMPORTANT NOTE: By default, htseq assumes your reads are stranded and will only count the reads that map in the same direction as the feature. If you have unstranded data or if you want to count reads mapping in all directions (maybe to detect antisense genes), use --stranded no option. If you have truseq data which uses dUTP method for creating stranded libraries, your reads will actually be in reverse direction when compared to the feature. So, use --stranded reverse.
htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C1_R1.sam ../reference/genes.formatted.gtf > C1_count1.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C1_R2.sam ../reference/genes.formatted.gtf > C1_count2.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C1_R3.sam ../reference/genes.formatted.gtf > C1_count3.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C2_R1.sam ../reference/genes.formatted.gtf > C2_count4.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C2_R2.sam ../reference/genes.formatted.gtf > C2_count5.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C2_R3.sam ../reference/genes.formatted.gtf > C2_count6.gff join C1_count1.gff C1_count2.gff| join - C1_count3.gff | join - C2_count4.gff |join - C2_count5.gff|join - C2_count6.gff > gene_counts_HTseq.gff #if you have many samples, use for-loop and join
Warning: To submit to queue
nano commands.htseq #put these lines in the commands file htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C1_R1.sam ../reference/genes.formatted.gtf > C1_count1.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C1_R2.sam ../reference/genes.formatted.gtf > C1_count2.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C1_R3.sam ../reference/genes.formatted.gtf > C1_count3.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C2_R1.sam ../reference/genes.formatted.gtf > C2_count4.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C2_R2.sam ../reference/genes.formatted.gtf > C2_count5.gff htseq-count -m intersection-nonempty --stranded reverse -i gene_id hisat_results/C2_R3.sam ../reference/genes.formatted.gtf > C2_count6.gff
launcher_creator.py -j commands.htseq -n htseq -q normal -t 02:00:00 -a UT-2015-05-18 -l htseq_launcher.slurm -m "module load htseq" sbatch htseq_launcher.slurm
AFTER THIS COMPLETES:
join C1_count1.gff C1_count2.gff| join - C1_count3.gff | join - C2_count4.gff |join - C2_count5.gff|join - C2_count6.gff > gene_counts_HTseq.gff #if you have many samples, use for-loop and join
Then take a peek at the results...
head gene_counts.gff wc -l gene_counts.gff #If you don't have your own results yet head gene_counting_results/gene_counts.gff wc -l gene_counting_results/gene_counts.gff
head gene_counts_HTseq.gff wc -l gene_counts_HTseq.gff #If you don't have your own results yet head gene_counting_results/gene_counts_HTseq.gff wc -l gene_counting_results/gene_counts_HTseq.gff
HTseq-count is strand-specific in default. Therefore, read counts for each gene in gene_counts_HTseq.gff are approximately a half counts in gene_counts.gff for the corresponding gene.
Bedtools multicov output format:
Bedtools output file will be a tab-delimited file with all columns of the gtf/gff file (annotation file) followed by a count column for every input sample .
Columns for our output file are the following (the columns newly added by bedtools in bold):
chr/seqname source feature startposition endposition score strand frame attribute C1_R1count C1_R2count C1_R3count C2_R1count C2_R2count C2_R3count
HTSeq output format:
HTSeq output file (after concatenation) will be a tab-delimited file with the feature name followed by a count column or every input sample.
Columns for our output file are the following
Genename C1_R1count C1_R2count C1_R3count C2_R1count C2_R2count C2_R3count
Gene Counting If you Mapped to the Transcriptome
eXpress (https://pachterlab.github.io/eXpress/manual.html) is a feature quanitification tool that is useful if you mapped RNA-Seq to the transcriptome (and didn't use a pseudoaligner like kallisto).
module spider eXpress module load express type express
Warning: To submit to queue
nano commands.express
#put these commands into the file
singularity exec ${BIOCONTAINER_DIR}/biocontainers/express/express-1.5.1--h2d50403_1.simg express -o C1_R1_counts.express ../reference/transcripts.fasta hisat_results/C1_R1.sam
singularity exec ${BIOCONTAINER_DIR}/biocontainers/express/express-1.5.1--h2d50403_1.simg express -o C1_R2_counts.express ../reference/transcripts.fasta hisat_results/C1_R2.sam
singularity exec ${BIOCONTAINER_DIR}/biocontainers/express/express-1.5.1--h2d50403_1.simg express -o C1_R3_counts.express ../reference/transcripts.fasta hisat_results/C1_R3.sam
singularity exec ${BIOCONTAINER_DIR}/biocontainers/express/express-1.5.1--h2d50403_1.simg express -o C2_R1_counts.express ../reference/transcripts.fasta hisat_results/C2_R1.sam
singularity exec ${BIOCONTAINER_DIR}/biocontainers/express/express-1.5.1--h2d50403_1.simg express -o C2_R2_counts.express ../reference/transcripts.fasta hisat_results/C2_R2.sam
singularity exec ${BIOCONTAINER_DIR}/biocontainers/express/express-1.5.1--h2d50403_1.simg express -o C2_R3_counts.express ../reference/transcripts.fasta hisat_results/C2_R3.sam
launcher_creator.py -j commands.express -n express -q normal -t 04:00:00 -a UT-2015-05-18 -l express_launcher.slurm -m "module load biocontainers;module load express" sbatch express_launcher.slurm
Other Gene Counting Options
If you want to perform all above operations in R enviornment, GRanges (along with Rsamtools) is a useful option. An example vignette is available here. featureCounts is also a great option (http://bioinf.wehi.edu.au/featureCounts/). Kallisto is also a great transcript quantification option.
Let's look at how to check for differential expression now.