Reservations
Use our summer school reservation (CoreNGSday5) when submitting batch jobs to get higher priority on the ls6 normal queue today:
sbatch --reservation=CoreNGSday5 <batch_file>.slurm
idev -m 180 -N 1 -A OTH21164 -r CoreNGSday5
Overview
As we have seen, the SAMTools suite allows you to manipulate the SAM/BAM files produced by most aligners. There are many sub-commands in this suite, but the most common and useful are:
- Convert text-format SAM files into binary BAM files (samtools view) and vice versa
- Sort BAM files by reference coordinates (samtools sort)
- Index BAM files that have been sorted (samtools index)
- Filter alignment records based on BAM flags, mapping quality or location (samtools view)
Since BAM files are binary, they can't be viewed directly using standard Unix file viewers such as more, less and head. We have seen how samtools view can be used to binary-format BAM files into text format for viewing. But samtools view also has options that let you do powerful filtering of the output. We focus on this filtering capability in this set of exercises.
The most common samtools view filtering options are:
- -q N – only report alignment records with mapping quality of at least N (>= N).
- -f 0xXX – only report alignment records where the specified flags are all set (are all 1)
- you can provide the flags in decimal, or as here as hexadecimal
- -F 0xXX – only report alignment records where the specified flags are all cleared (are all 0)
Setup
Login to ls6.tacc.utexas.edu and start an idev session.
idev -m 180 -N 1 -A OTH21164 -r CoreNGSday5
Next set up a directory for these exercises, and copy an indexed BAM file there. This is a renamed version of the yeast_pairedend.sort.bam file from our Alignment workflow exercises.
mkdir -p $SCRATCH/core_ngs/samtools cd $SCRATCH/core_ngs/samtools cp $CORENGS/yeast_stuff/*.bam* .
References
Handy links
- The SAM format specification
- especially section 1.4 - alignment section fields
- Manual for SAMTools
- especially the 1st section on samtools view.
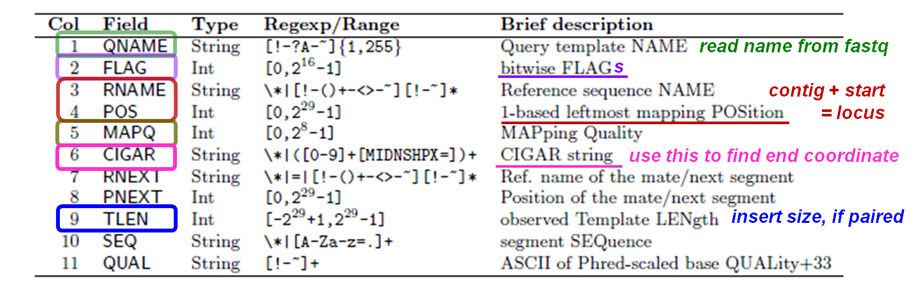
SAM header fields
The 11 SAM alignment record required fields (Tab-delimited).
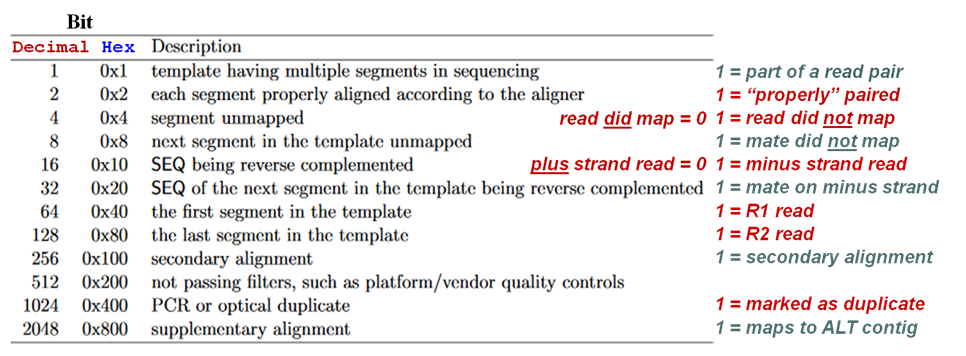
SAM flags field
Meaning of each bit (flag) in the SAM alignment records flags field (column 2). The most commonly flags are denoted in red.
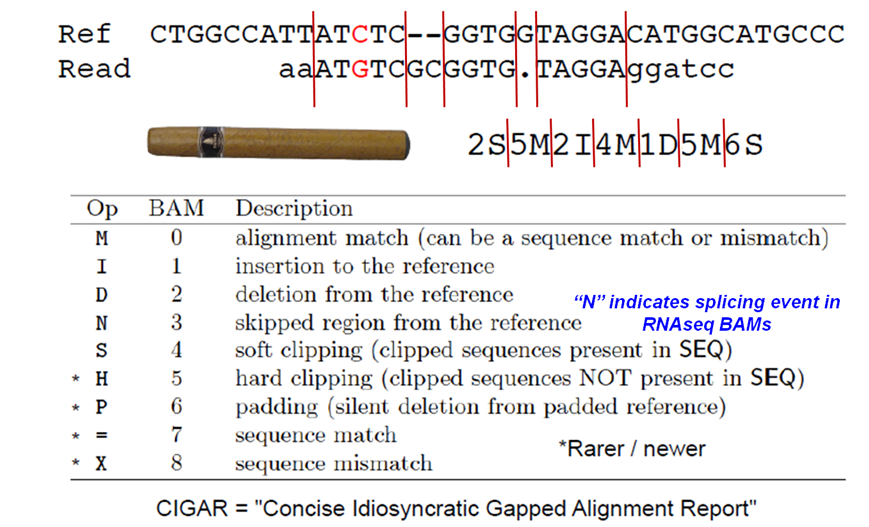
SAM CIGAR string
Format of the CIGAR string in column 6 of SAM alignment records.
Exercises
Analyzing the CIGAR string for indels
Suppose we want to know how many alignments included insertions or deletions (indels) versus the reference. Looking at the CIGAR field definition table above, we see that insertions are denoted with the character I and deletions with the character D. We'll use this information, along with samtools view, cut and grep, to count the number of indels.
We'll do this first exercise one step at a time to get comfortable with piping commands. Start by just looking at the first few alignment records of the BAM file:
module load biocontainers # takes a while module load samtools cd $SCRATCH/core_ngs/samtools samtools view yeast_pe.sort.bam | head
With all the line wrapping, it looks pretty ugly. Still, you can pick out the CIGAR string in column 6. Let's select just that column with cut:
samtools view yeast_pe.sort.bam | cut -f 6 | head -20
Next, make sure we're only looking at alignment records that represent mapped reads. The -F 0x4 option says to filter records where the 0x4 flag (read unmapped) is 0, resulting it only mapped reads being output.
samtools view -F 0x4 yeast_pe.sort.bam | cut -f 6 | head -20
Now we use grep with the pattern '[ID]' to select lines (which are now just CIGAR strings) that have Insert or Deletion operators. The brackets ( [ ] ) denote a character class pattern, which matches any of the characters inside the brackets. Be sure to ask for Perl regular expressions (-P) to be sure that this character class syntax is recognized.
samtools view -F 0x4 yeast_pe.sort.bam | cut -f 6 | grep -P '[ID]' | head
Ok, this looks good. We're ready to run this against all the alignment records, and count the results:
samtools view -F 0x4 yeast_pe.sort.bam | cut -f 6 | grep -P '[ID]' | wc -l
There are 6697 such records.
What is that in terms of the rate of indels? For that we need to count the total number of mapped reads. Here we can just use the -c (count only) option to samtools view.
samtools view -c -F 0x4 yeast_pe.sort.bam
There should be 547664 mapped alignments.
Knowing these two numbers we can just divide them, using awk (remember, bash only does integer arithmetic). Because we're not piping anything in to awk, any body we specify won't be executed. So we do the math in the BEGIN section:
awk 'BEGIN{ print 100*6697/547664,"%" }'
The result should be 1.22283 %.
In addition to the rate, converted to a percentage, we also output the literal percent sign ( % ). The double quotes ( " ) denote a literal string in awk, and the comma between the number and the string says to separate the two fields with whatever the default Output Field Separator (OFS) is. By default, both awk's Input Field Separator (FS) is whitespace (any number of space and Tab characters) and its Output Field Separator is a single space.
So what if we want to get fancy and do all this in a one-liner command? We can use echo and backtick evaluation to put both numbers in a string, then pipe that string to awk. This time we use awk body code, and refer to the two whitespace-separated fields being passed in: total count ($1) and indel count ($2).
echo "`samtools view -F 0x4 -c yeast_pe.sort.bam` `samtools view -F 0x4 yeast_pe.sort.bam | cut -f 6 | grep -P '[ID]' | wc -l`" | awk '{ print 100 * $2/$1,"%" }'
awk also has a printf function, which can take the standard formatting commands (see https://en.wikipedia.org/wiki/Printf_format_string#Type_field).
awk 'BEGIN{ printf("%.2f %%\n", 100*6697/547664) }'
Notes:
- The printf arguments are enclosed in parentheses since it is a true function.
- The 1st argument is the format string, enclosed in double quotes.
- The %.2f format specifier says to output a floating point number with 2 digits after the decimal place.
- The %% format specifier is used to output a single, literal percent sign.
- Unlike the standard print statement, the printf function does not automatically append a newline to the output, so \n is added to the format string here.
- Remaining arguments to printf are the values to be substituted for the format specifiers (here our percentage computation).
Filtering by location range
Sometimes you just want to examine a subset of reads in detail. Once you have a sorted and indexed BAM, you can use the coordinate filtering options of samtools view to do this. Here are some examples:
When you're interested in mapped reads (which is true most of the time) be sure to specify the -F 0x4 option, which says to filter records where the 0x4 flag (read unmapped) is 0, resulting it only mapped reads being output.
cd $SCRATCH/core_ngs/samtools # count the number of reads mapped to chromosome 2 (chrII) samtools view -c -F 0x4 yeast_pe.sort.bam chrII # count the number of reads mapped to chromosomes 1 or M (chrI, chrM) samtools view -c -F 0x4 yeast_pe.sort.bam chrI chrM # count the number of reads mapped to chromosomes 1 that overlap coordinates 1000-2000 samtools view -c -F 0x4 yeast_pe.sort.bam chrI:1000-2000 # since there are only 20 reads in the chrI:1000-2000 region, examine them individually samtools view -F 0x4 yeast_pe.sort.bam chrI:1000-2000 # look at a subset of field for chrI:1000-2000 reads # 2=flags, 3=contig, 4=start, 5=mapping quality, 6=CIGAR, 9=insert size samtools view -F 0x4 yeast_pe.sort.bam chrI:1000-2000 | cut -f 2-6,9
samtools view -L <bed_file>
You can also provide a BED-format file with one record for each desired overlap region: samtools view -L <bed_file>. This is a quick way to perform one of the functions of bedtools intersect.
Since you will find yourself wanting to interpret the flags field, and it's easier to do that when it is represented as hexadecimal, let's use awk to help do that.
# look at a subset of field for chrI:1000-2000 reads
# 2=flags, 3=contig, 4=start, 5=mapping quality, 6=CIGAR, 9=insert size
samtools view -F 0x4 yeast_pe.sort.bam chrI:1000-2000 | cut -f 2-6,9 | \
awk 'BEGIN{FS=OFS="\t"}{$1 = sprintf("0x%x", $1); print}'
Notes:
- If a command line is continued on more than one line, the line continuation character ( \ ) is used.
- There is a sprintf (string print formatted) function in awk, just as there is in many modern programming languages.
- We use sprintf to re-format the 1st input field (here the cut flags) in hex
- using the format directive (%x)
- also adding a literal "0x" prefix to denote the numeric base is hexadecimal
- The results of sprintf are stored back into the 1st field ($1), replacing the original value.
- awk's print statement is then used with no other arguments to print all its input fields, including the changed $1.
Exercise: How many of the chrI:1000-2000 alignments are from properly paired mapped reads?
About mapping quality
Mapping qualities are a measure of how likely a given sequence alignment to its reported location is correct. If a read's mapping quality is low (especially if it is zero, or mapQ 0 for short) the read maps to multiple locations on the genome (they are multi-hit or multi-mapping reads), and we can't be sure whether the reported location is the correct one.
Aligners also differ in whether they report alternate alignments for multi-hit reads. Some things to keep in mind:
- Alternate locations for a mapped read are are flagged as secondary (flag 0x100)
- While they often provide valuable information, secondary reads must be filtered for some downstream applications
- e.g., ChIP-seq peak calling and variant analysis with GATK.
- When secondary reads are reported, the total number of alignment records in the BAM file is greater than the number of reads in the input FASTQ files!
- this affects how the true mapping rate must be calculated
- true mapping rate = (pirmary mapped reads) / (total BAM file sequences - secondary mapped reads)
Here are some examples of how different aligners handle reporting of multi-hit reads and their mapping qualities:
- bwa aln (global alignment) and bowtie2 with default parameters (both --local default end-to-end mode) report at most one location for a read that maps
- this will be the location with the best mapping quality and alignment
- if a given read maps equally well to multiple locations, these aligners pick one location at random
- bwa aln will always report a 0 mapping quality for these multi-hit reads
- bowtie2 will report a low mapping quality (< 10), based on the complexity (information content) of the sequence
- bwa mem (local alignment) can always report more than one location for a mapped read
- its definition of a secondary alignment is different (and a bit non-standard)
- if one part of a read maps to one location and another part maps somewhere else (e.g. because of RNA splicing), the longer alignment is marked as primary and the shorter as secondary.
- there is no way to disable reporting of secondary alignments with bwa mem.
- but they can be filtered from the sorted BAM with -F 0x100 (secondary alignment flag = 0).
- its definition of a secondary alignment is different (and a bit non-standard)
- bowtie2 can be configured to report more than one location for a mapped read
- the -k N option says to report up to N alignments for a read
- most transcriptome-aware RNA-seq aligners by default report more than one location for a mapped read
- e.g. hisat2, star, tophat2.
- when reads are quantified (counted with respect to genes), multiply-mapped reads can be counted fractionally
- e.g. if a read maps to 5 genes, it can be counted as 1/5 for each of the genes
Filtering high-quality reads
Using our yeast_pe.sort.bam file, let's do some some quality filtering.
Exercise: Use samtools view with -F, -f and -q options to create a BAM containing only mapped, properly paired, high-quality (mapQ 20+) reads. Call the output file yeast_pe.sort.filt.bam.
Exercise: How many records are in the filtered BAM compared to the original? How many read pairs does this represent?
Exercise: How many primary aligned reads (0x100 = 0) are in the bwa_local.sort.dup.bam file?
Combining SAM flags
samtools view only pays attention to one -F or -f option, so to specify more than one flag value you need to combine them into one number. For example, combining 0x100 and 0x4 yields 0x104.