In this lab, you will explore a popular new transcriptome-aware mapper called HISAT2. Simulated RNA-seq data will be provided to you; the data contains paired-end reads that have been generated in silico to replicate real gene count data from Drosophila. The data simulates two biological groups with three biological replicates per group (6 samples total). The objectives of this lab is to:
12 raw data files have been provided for all our further RNA-seq analysis:
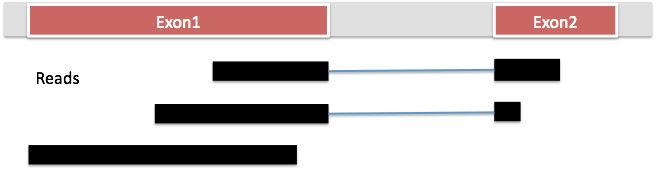
HISAT2 is the fastest spliced mapper currently available. It is part of the new tuxedo suite of tools and it will map RNA-Seq data to the genome as well as identify splice junctions. HISAT2, like BWA and bowtie, uses burrows-wheeler transform (BWT) to compress genomes such that they require very little memory to store. Like BWA and bowtie, it builds indexes out of the transformed genomes using a special scheme called FM indexing. This makes it possible to search through these genomes rapidly. Unlike BWA and bowtie, HISAT2 builds a whole genome global index and tens of thousands of small local indexes to make spliced alignment possible. Despite the many indexes, because it uses BWT and FM indexing, the indexes take a very small memory footprint (~5gb RAM for the whole human genome), making it possible to run hisat2 on a standard laptop.
With the human genome, for example, hisat2 builds one global index and 48000 local indexes (each 64000bp long). The size of the local indexes is large enough that 90% of introns will fall into a single local index (on average, human introns are >6kb long).
First, the longer part of a read that maps to the genome contiguously (called the anchor) is mapped using the global index. Once this is mapped, this helps to to identify the relevant local index. HISAT can usually align the remaining part of the read (small anchor) within a single local index rather than searching across the whole genome.

First, make sure you are in the right directory for this exercise.
cds cd my_rnaseq_course cd day_2/hisat_exercise ls |
module load biocontainers module spider hisat2 module load hisat2/ctr-2.1.0--py36pl5.22.0_0 |
hisat2 |
This doesn't work because when you load biocontainer (singularity) modules, it doesn't simply add the tool/executable to your path. Instead, it defines a function and you will needd to use that function definition to run the tool.
type hisat2 |
Part 1. Create a index of your reference
NO NEED TO RUN THIS NOW- YOUR INDEX HAS ALREADY BEEN BUILT!
singularity exec ${BIOCONTAINER_DIR}/biocontainers/hisat2/hisat2-2.1.0--py36pl5.22.0_0.simg hisat2-build reference/genome.fa reference/genome.fa |
Part 2. Align the samples to reference using hisat2
Create a
|
Hisat2 output
1.SAM file : HISAT2 alignment output in standard SAM format.
2.Junctions file : File containing all detected junctions with the format:
chr startpos endpos orientation
3. Log file : The log file should contain alignment summaries.
ls results head results/GSM794483_C1.sam head results/GSM794483_C1.junctions |
11607353 reads; of these:
11607353 (100.00%) were paired; of these:
21592 (0.19%) aligned concordantly 0 times
11417720 (98.37%) aligned concordantly exactly 1 time
168041 (1.45%) aligned concordantly >1 times
----
21592 pairs aligned concordantly 0 times; of these:
82 (0.38%) aligned discordantly 1 time
----
21510 pairs aligned 0 times concordantly or discordantly; of these:
43020 mates make up the pairs; of these:
25009 (58.13%) aligned 0 times
9694 (22.53%) aligned exactly 1 time
8317 (19.33%) aligned >1 times
99.89% overall alignment rate |
BACK TO COURSE OUTLINE