Objectives
Once we've obtained abundance counts for our genes/exons/transcripts, we are usually interested in identifying those genes/exons/transcripts that are differentially expressed.
In this section, you will learn about different tools for identifying differentially expressed genes from gene count data. The same data from the previous exercises will be used. The data contains 75 bp paired-end reads that have been generated in silico to replicate real gene count data from Drosophila. The data simulates two biological groups with three biological replicates per group (6 samples total).
- Learn about DESeq2, DEXSeq and cuffdiff packages and the differences among these packages.
- Become familiar with basic R usage and installing Bioconductor modules.
- Learn how to use DESeq2 to identify differentially expressed genes.
- Learn how to use cuffdiff pacakge to identify differentially expressed genes.
Get set up
| Code Block | ||
|---|---|---|
| ||
cds cd my_rnaseq_course cd day_3_partA/gene_expression_exercise # you should have already copied this over |
Let's first start installing some R tools because they may take a while.
| Warning |
|---|
When following along here, please switch to your idev session for running these example commands. |
| Code Block | ||
|---|---|---|
| ||
ssh <username>@ls5.tacc.utexas.edu idev -m 120 -q development -A UT-2015-05-18 -r CCBB_Day_3 |
Introduction
Most RNA-Seq experiments are conducted with the aim of identifying genes/exons that are differentially expressed between two or more conditions. Many computational tools are available for performing the statistical tests required to identify these genes/exons.
Simply put, all these tools do three steps (and they can vary in how they do these steps):
- Normalization of gene counts
- Represent the gene counts by a distribution that defines the relation between mean and variance (dispersion).
- Perform a statistical test for each gene to compare the distributions between conditions.
- Provide fold change, P-value information, false discovery rate for each gene.
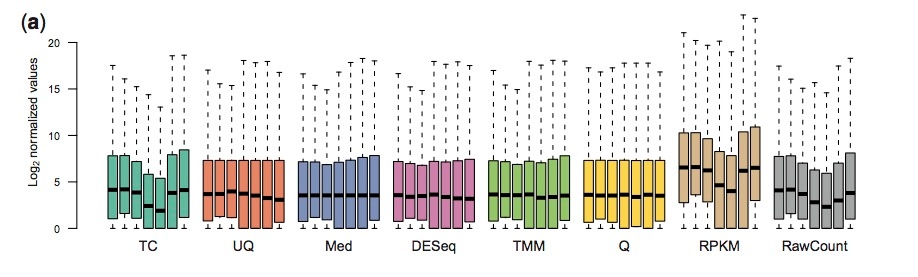
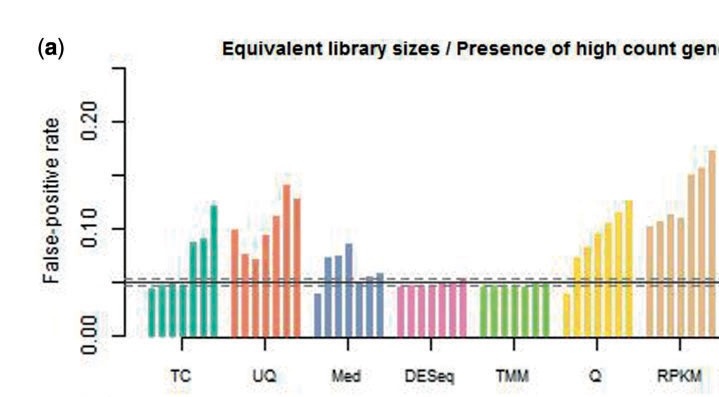
Why Normalize?
Normalization smooths out technical variations among the samples we are comparing so that we can more confidently attribute variations we see to biological reasons.
We usually normalize for:
- Sequencing depth: Say we are comparing gene counts in sample A against sample B. If you start out with 10 million reads in sample A vs 1 million reads in sample B, a 10 fold increase in expression in sample A is going to be purely due to its sequencing depth.
- Gene length: A gene that is twice as long is likely to have twice as many reads sampling it.
From: Dillies A et al, A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis,
doi:10.1093/bib/bbs046 .
Most commonly done normalization
RPKM: Normalizes for sequencing depth and gene length.
RPKM = reads per kilobase per million mapped reads
RPK= No.of Mapped reads/ length of transcript in kb (transcript length/1000)
RPKM = RPK/total no.of reads in million (total no of reads/ 1000000)
| DESeq2 | edgeR | DEXSeq | Ballgown | |
|---|---|---|---|---|
| Normalization | Median scaling size factor | TMM | Median scaling size factor | FPKM , but also has provisions for others |
| Distribution | Negative binomial | Negative binomial | Negative binomial | Negative binomial |
| DE Test | Negative binomial test | Fisher exact test | Modified T test | T test |
| Advantages | Straightforward, fast, DESeq2 allows for complicated study designs, with multiple factors | Straightforward, fast, good with small number of replicates. | Good for identifying exon-usage changes | Good for identifying isoform-level changes, splicing changes, promotor changes. Not as straightforward, somewhat of a black box |
R and Bioconductor, very briefly...
R is a very common scripting language used in statistics. There are whole courses on using R going on in other SSI classrooms as we speak! Inside the R universe, you have access to an incredibly large number of useful statistical functions (Fisher's exact test, nonlinear least-squares fitting, ANOVA ...). R also has advanced functionality for producing plots and graphs as output.
Regrettably, R is a bit of it's own bizarro world, as far as how its commands work. (Futhermore, Googling "R" to get help can be very frustrating.) The conventions of most other programming and scripting languages seem to have been re-invented by someone who wanted to do everything their own way in R. Just like we write shell scripts in bash, you can write R scripts that carry out complicated analyses.
| Warning | ||
|---|---|---|
| ||
They are the R prompt to remind you which commands are to be run inside the R shell! |
| Code Block | ||
|---|---|---|
| ||
module spider Rstats module load Rstats module load RstatsPackages/3.5.1 |
Hints for working with R
- Don't forget: it's
q()to quit. - For help with a function, type
?command. Try?read.table. Theqkey gets you out of help, just like for amanpage. - The left arrow
<-(less-than-dash) is the same as an equals sign=. You can use them interchangeably. - The prompt we will sometimes be showing for R is
>. Don't type this for a command. It is like thelogin1$at the beginning of the bash prompt when you log in to Stampede2. It just means that you are in theRshell.
You can type the name of a variable to have its value displayed. Like this...
| Code Block |
|---|
R
#Where are you in R- this is your working directory
getwd()
#Change working directory
setwd(mydirectory)
x <- 10 + 5 + 6
x
[1] 21
#Vectors- single data type
id <- c(1,2,3,4,5)
sex<- c("male","male","female","female","female")
id
sex
#Lists-multiple data types
x<-list(1:3, c("Dave", "Van", "Lesa"), c("male","male","female"))
x
str(x)
#Factors- predefined categorial variables
vfact <- factor(sex)
vfact
#Data frames - most tables get read into as data frames-lots of equal length vectors.
mydata <- data.frame(id,sex)
mydata
names(mydata) <- c("Person's ID","Person's sex")
mydata
#Functions to look at data structures
length(id)
typeof(id)
str(x)
summary(mydata)
class(mydata)
names(mydata)
#Reading data from files
read.table()
?read.table()
#Writing data into files
write.table()
#To see all the objects we have available
ls()
#to save all your objects and your command history within R
save.image(file="test.Rdata")
savehistory(file="test.Rhistory")
#when you reenter R, load already created objects and commands
load(file="test.Rdata")
loadhistory(file = "test.Rhistory")
#to quit out of R
q()
#to run a R script from the command line
R CMD BATCH <rscript> |
Bioconductor packages for R
Like other languages, R can be expanded by loading packages. The R equivalent of Bioperl or Biopython is Bioconductor. Bioconductor can theoretically do things for you like convert sequences (none of us use it for that), but where it really shines is in doing statistical tests (where is it second-to-none in this list of languages). Many functions for analyzing microarray data are implemented in R, and this strength has now carried over to the analysis of RNAseq data.
Here's how you install two modules that we will need for this exercise: (But first, remember that to get R on stampede2, you'll need to load the R module or have a version of R installed locally)
| Warning |
|---|
The install commands may take several minutes to complete. |
| Code Block | ||
|---|---|---|
| ||
#These commands will work for any Bioconductor package!
R
#within R
source("https://bioconductor.org/biocLite.R")
biocLite("BiocUpgrade")
#It will now prompt you if you want to update all packages. Say yes. It will ask you if you want to install R libraries into your personal/local R library. Say yes to this as well.
biocLite("DESeq2")
biocLite("readr")
biocLite('tximport')
biocLite('rhdf5') |
When you start R later, you will not need to re-install the modules. You can load them with just these commands:
| Code Block | ||
|---|---|---|
| ||
login1$ R
library('DESeq2')
library('readr')
library('tximport')
library('rhdf5')
|
DESeq2
Input:
DESeq2 takes as input count data in several forms: a table form, with each column representing a biological replicate/biological condition. DESEQ2 can also read data directly from htseq results, so we can use the 6 files we generated using htseq as input for DESeq2. The count data must be raw counts of sequencing reads, not already normalized data.
Example:
C1_R1 C1_R2 C1_R3 C2_R1 C2_R2 C2_R2
FBgn0000003 0 0 0 0 0 0
FBgn0000008 92 161 76 70 140 88
FBgn0000014 5 1 0 0 4 0
DESeq2 (with the use of an additional packages called tximport and readr) can read data directly from kallisto abundance files. We will need to provide the location of the 6 abundance files, the sample names associated to each file and a Sample Table that gives the mapping between sample and condtion.
SampleName Condition
DESeq2 Scripts:
- DESeq2 script to work with kallisto count output is provided below.
| Code Block | ||
|---|---|---|
| ||
#load libraries
library(tximport)
library(readr)
library("DESeq2")
library('rhdf5')
#Import a file called file_list with all the locations of the abundance.tsv files
#eg below:
#/stor/SCRATCH/sample1/abundances.tsv
#/stor/SCRATCH/sample2/abundances.tsv
#/stor/SCRATCH/sample3/abundances.tsv
#/stor/SCRATCH/sample4/abundances.tsv
files<-as.character(read.table("file_list", header=FALSE)$V1)
#Import a file called samples with the sample names corresponding to each file in the file_list
#eg below:
#sample1
#sample2
#sample3
#sample4
#look at the data structures
files
samples<-as.character(read.table("samples",header=FALSE)$V1)
names(files)<-samples
#look at the data structures
samples
files
#Import a file called sampletable which is a tab-delimited file that contains each samplename along with the condition
#eg below:
#samples condition
#sample1 alc
#sample2 alc
#sample3 con
#sample4 con
sampleTable <-read.table("sampleTable",header=TRUE, row.names=1)
#look at the data structure
head(sampleTable)
#IMPORTANT: MAKE SURE THE SAMPLES AND FILE_LIST ARE IN THE SAME ORDER- SAMPLES SHOULD MATCH UP WITH FILES
samples==rownames(sampleTable) #should return TRUE for all
#Import a file called tx2gene.csv which a csv file that contains the transcript id to gene id mapping
#For Drosophila, this is located at: tx2gene.csv
tx2gene <- read.csv("tx2gene.csv")
#look at this data structure
#read in kallisto abundance files, summarizing by gene
txi <- tximport(files, type = "kallisto", tx2gene = tx2gene)
names(txi)
#make a deseq2 object from the kallisto summarized counts
ddsMatrix <- DESeqDataSetFromTximport(txi, sampleTable, ~condition)
ddsMatrix
#Optionally, if you want to save this count matrix as a file
write.csv(assay(ddsMatrix), file="genecounts.raw.csv")
#Optionally, if you want to do variance stabilizing transformation or regularized log transformation on this count matrix and then save as a file: This can become input to things like wgcna, pca
vsd<- vst(ddsMatrix)
write.csv(assay(vsd), file="genecounts.variancestabilized.csv")
#estimate size factors, dispersion, normalize and perform negative binomial test to compare across conditions
dds<-DESeq(ddsMatrix)
#collect results from the statistical testing, order by adjusted pvalue and write into an output file
res<-results(dds)
resOrdered <- res[order(res$padj),]
summary(res)
write.csv(resOrdered, "deseq2_kallisto_C1_vs_C2.csv")
#generate MA plot
pdf('MAPlot.pdf')
plotMA(dds,ylim=c(-2,2),main="DESeq2")
dev.off()
#save the R data and history
save.image(file="deseq2.kallisto.Rdata")
savehistory(file="deseq2.kallisto.Rhistory") |
| Code Block | ||
|---|---|---|
| ||
R CMD BATCH deseq2.kallisto.R |
2. DESeq2 script to work with Htseq count output
| Code Block | ||
|---|---|---|
| ||
library("DESeq2")
#GET HTSEQ COUNTS AND SET UP SAMPLE TABLE
directory<-(getwd())
samples <- c("C1_count1.gff", "C1_count2.gff", "C1_count3.gff", "C2_count4.gff", "C2_count5.gff", "C2_count6.gff")
conditions <- c("control", "control", "control", "treated","treated","treated")
sampleTable<-data.frame(sampleName=samples, fileName=samples, condition=conditions)
sampleTable
#BUILD A DESEQ2 OBJECT FROM THE HTSEQ COUNTS DATA
ddsHTSeq<-DESeqDataSetFromHTSeqCount(sampleTable=sampleTable, directory=directory, design=~condition)
colData(ddsHTSeq)$condition<-factor(colData(ddsHTSeq)$condition, levels=c("control", "treated"))
#LOOK AT THE DESEQ2 OBJECT WE'VE CREATED BY READING IN HTSEQ COUNT FILES
ddsHTSeq
#RUN THE STATISTICAL TEST IN ONE GO- NORMALIZATION, ESTIMATE DISPERSION/VARIANCE AND DO TEST FOR DIFFERENTIAL EXPRESSION
dds<-DESeq(ddsHTSeq)
res<-results(dds)
res<-res[order(res$padj),]
mcols(res,use.names=TRUE)
summary(res)
#GENERATE MA PLOT
jpeg('MAPlot_htseq.jpg')
plotMA(dds,ylim=c(-2,2),main="DESeq2")
dev.off()
#WRITE RESULTS INTO FILE
write.csv(as.data.frame(res),file="deseq2_htseq_C1_vs_C2.csv") |
| Code Block | ||
|---|---|---|
| ||
#IF YOU DIDNT WANT TO RUN THE SCRIPT INTERACTIVELY R CMD BATCH deseq2.htseq.R |
Lets look at our results
| Code Block | ||
|---|---|---|
| ||
head results/deseq2_kallisto_C1_vs_C2.csv |
Find the top 10 upregulated genes
| Code Block | ||
|---|---|---|
| ||
#DESeq2 results sed 's/,/\t/g' results/deseq2_kallisto_C1_vs_C2.csv|sort -n -r -k3,3|cut -f 1,3|head #Notice the idiosyncracy with sort sed 's/,/\t/g' results/deseq2_kallisto_C1_vs_C2.csv|sort -n -r -k3,3|grep -v 'e-0'|cut -f 1,3|head |
Find the top 10 downregulated genes
| Code Block | ||
|---|---|---|
| ||
#DESeq2 results sed 's/,/\t/g' results/deseq2_kallisto_C1_vs_C2.csv|sort -n -k3,3|cut -f 1,3|head #Notice the idiosyncracy with sort sed 's/,/\t/g' results/deseq2_kallisto_C1_vs_C2.csv|sort -n -k3,3|grep -v 'e-0'|cut -f 1,3|head |
2. Select DEGs with following cut offs- Fold Change >=2 (or <= -2) (this means log 2 fold change >= 1 or <=-1) and adj p value < 0.05 and count how many DEGs we have
| Code Block | ||
|---|---|---|
| ||
#DESeq2 results
sed 's/,/\t/g' results/deseq2_kallisto_C1_vs_C2.csv|awk '{if ((($3>=1)||($3<=-1))&&($7<=0.05)) print $1,$3,$7}'|wc -l
|
If you wanted to use DESeq2 for more complicated designs (with multiple factors, multiple levels), you can by adjusting two things: design and contrast.
Advanced options
#One factorddsHTSeq<-DESeqDataSetFromHTSeqCount(sampleTable=sampleTable, directory=directory, design=~condition) #Two factors (one factor has multiple levels): condition (control, treated), and sequencing type (single, paired, matepair)ddsHTSeq<-DESeqDataSetFromHTSeqCount(sampleTable=sampleTable, directory=directory, design=~type + condition) #Fold changes will by default be provided for condition (treated vs control)res <- results(dds)#To view fold changes for sequencing type, use contrastres <- results(dds, contrast = c("type", "paired", "single"))res <- results(dds, contrast = c("type", "matepair", "single")) |
DEXSeq
This package is meant for finding differential exon usage between samples from different conditions.
Relative usage of an exon = transcripts from the gene that contain this exon / all transcripts from the gene
For each exon (or part of an exon) and each sample :
- count how many reads map to this exon
- count how many reads map to other exons of the same gene.
- calculate ratio of 1 to 2.
- Look for changes in this ratio across conditions
- Look for statistically significant changes in this ratio across conditions, by using replicates.
This lets you identify changes in alternative splicing, changes in usage of alternative transcript start sites.
Ballgown
Ballgown (a part of the new tuxedo suite) is a popular tool for testing for differential expression. We will cover this along with the rest of the tuxedo suite.
BACK TO COURSE OUTLINE